
Полезное:
Как сделать разговор полезным и приятным
Как сделать объемную звезду своими руками
Как сделать то, что делать не хочется?
Как сделать погремушку
Как сделать так чтобы женщины сами знакомились с вами
Как сделать идею коммерческой
Как сделать хорошую растяжку ног?
Как сделать наш разум здоровым?
Как сделать, чтобы люди обманывали меньше
Вопрос 4. Как сделать так, чтобы вас уважали и ценили?
Как сделать лучше себе и другим людям
Как сделать свидание интересным?

Категории:
АрхитектураАстрономияБиологияГеографияГеологияИнформатикаИскусствоИсторияКулинарияКультураМаркетингМатематикаМедицинаМенеджментОхрана трудаПравоПроизводствоПсихологияРелигияСоциологияСпортТехникаФизикаФилософияХимияЭкологияЭкономикаЭлектроника

Выбор НПВС с учетом риска лекарственных осложнений
|
|
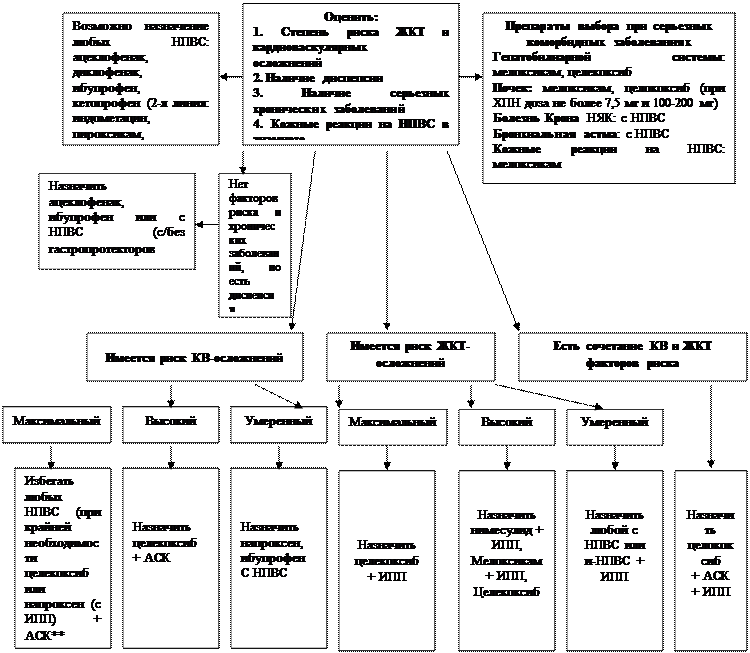
* Могут быть назначены с НПВС (на усмотрение лечащего врача)
** Ацетилсалициловая кислота
 Цитировано по «Применение нестероидных противовлспалительных средств. Клинические рекомендации [4].
Цитировано по «Применение нестероидных противовлспалительных средств. Клинические рекомендации [4].
ЛИТЕРАТУРА
1. Дзяк Г.В., Викторов А.П., Гришина Е.И. Нестероидные противовоспалительные препараты. – МОРИОН. Киев. – 1999. – 111с.
2. Каратеев А.Е., Насонов Е.Л., Корешков Г.Г. НПВС–индуцированная диспепсия: распространенность и возможность медикаментозной коррекции. Науч. практ. ревматол., 2003, 5, 76–78.
3. Каратеев А.Е., Насонова В.А. Развитие и рецидивирование язв желудка и двенадцатиперстной кишки у больных, принимающих нестероидные противовоспалительные препараты: влияние стандартных факторов риска. Тер. архив, 2008, 5, С.62–66.
4. Каратеев А.Е., Яхно Н.Н., Лазебник Л.Б. и др. Применение нестероидных противовоспалительных препаратов. Клинические рекомендации. Москва, ИМА–ПРЕСС, 2009, 167с.
5. Насонов Е.Л. Нестероидные противовоспалительные препараты: стандарты лечения // РМЖ. – 2001. - №9, 7-8. – С. 265-270.
6. Насонов Е.Л. Нестероидные противовоспалительные препараты (Перспективы применения в медицине). – М., «Анко», 2000. – 143с.
7. Насонов Е.Л., Лазебник Л.Б., Беленков Ю.Н. и сотр. Применение нестероидным противовоспалительных препаратов. Клинические рекомендации. Москва, «Алмаз», 2006, 88с.
8. Рациональная фармакотерапия ревматических заболеваний. Руководство для врачей под ред. В.А. Насоновой, Е.Л. Насонова. Гл.1. Нестероидные противовоспалительные препараты. – М., «Литтерра». – 2003. – С. 22-33.
9. Страчунский Л.С., Козлов С.Н. Нестероидные противовоспалительные средства. – Смоленск. – 1997. – 70с.
10. Arya N., Hawe M., Ozo C. Diclofenac suppositories and acute ischaemic proctitis. Ulster Med J. 2004, 73(1): 63–64.
11. Bennet A. Clinical importance of the multifoctorial actions of nimesulide. Drugs of Today 2001; 37 (Suppl. B): 9-14.
12. Bennet A., Villa G. Nimesulid: an NSAID that preferentially inhibits COX-2, and nas various unigue pharmacological activities. Exp. Opin. Pharmacotherapy. - 2000; 1: 277-286.
13. Bonnet C., Walsh D. Osteoarthritis, angiogenesis and inflammation. Rheumatology, 2005; 44(1):7—16.
14. Brooks P.M., Day R.O. Nonsteroidal antiinfla mmatory drugs – differences and similarities // N. Engl. J. Med., 1991, 324:1716-1725.
15. Brown C., Moodie J., Dickie G., et al. Analgesic efficacy and safety of single–dose oral and intramuscular ketorolac tromethamine for postoperative pain. Pharmacotherapy. 1990, 10, 59–70.
16. Champion G.D, Feng P.H„ Azuma T. et al. NSAID-induced gastrointestinal damage // Drugs, 1997, 53: 6-19.
17. Chan F., Hung L., Suen B. et al. Celecoxib versus diclofenac and omeprasole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med 2002, 947, 2104–2110.
18. Chandrasekharan N. V., Hu Dai, Lamar Turepu Roos K., Nathan K., Evanson., et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression PNAS published September 19, 2002, 10. 1073/pnas 162468699.
19. Cheung R., Krishnaswami S., Kowalski K. Analgesic efficacy of celecoxib in postoperative oral surgery pain: a single–dose, two–center, randomized, double–blind, active– and placebo–controlled study. Clin Ther. 2007; 29, Suppl: 2498–2510.
20. Davis M., Walsh D., LeGrand S., Naughton M. Symptom control in cancer patients: the clinical pharmacology and therapeutic role of suppositories and rectal suspensions. Support Care Cancer. 2002, 10(2): 117–138.
21. Dequerker J, Hawkey C, Kahan A, et al. Improvement in gastrointestinal tolerability of selective cyclooxyenase (COX)–2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large Scale Evaluation of COX inhibiting Therapies (SELECT) trial in osteoarthritis. Br J Rheumatol 1998; 37: 946–951.
22. Dougados M., Strand V., Simon L., Sands G., Bhadra P. Continuous versus intermittent celecoxib dosing in the treatment of osteoarthritis. ARD, 2009.
23. Emery P., Zeidler H., Kvien T., et al. Celecoxib versus diclofenac in long–term management of rheumatoid arthritis: randomized double–blind comparison. Lancet 1999; 354: 2106–2111.
24. Gizzi G., Villani V., Brandi G., et al. Ano–rectal lesions in patients taking suppositories containing non–steroidal anti–inflammatory drugs (NSAID). Endoscopy. 1990, 22(3): 146–158.
25. Goldstein J., Aisenbeg J., Berger M., et al. Effects of concominant aspirin (81 mg) оn incidence the of gastric and/or duodenal ulcers in healthy subjects taking celecoxib or naproxen: a randomized placebo–controlled trial. Ann Rheum Dis 2006; 65 (Suppl. II): 229 (TH0327 abst).
26. Greenwald M., Peloso P., Hasler F. et al. Etoricoxib improves pain and function in rheumatoid arthritis patients on background biologic therapy. A&R, 2009, 60 (10), suppl.,606.
27. Guslandi M. Gastric toxicity of antiplatelet therapy with low-dose aspirin // Drugs, 1997, 53: 1-5.
28. Hawkey C. et al. Gastrointestinal tolerability of meloxicam compared diclofenac in osteoarthritis patients. International MELISSA Study Group. Meloxicam Large–scale International Study Safety Assessment. Br J Rheumat 1998; 37: 1142–1147.
29. Hawkey C., Svoboda P., Fiedorowicz–Fabrycy I., et al. Gastroduodenal safety and tolerability of lumiracoxib compared with Ibuprofen and celecoxib in patients with osteoarthritis. J Rheumatol. 2004, 31(9): 1804–1810.
30. Helin–Salmivaara A., Virtanen A., Vesalainen R., et al. NSAID use and the risk of hospitalization for first myocardial infarction in the general population: a nationwide case–control study from Finland. Eur Heart J., 2006, 27(14), 1657–1663.
31. Hаskinsson E.C., Irani M., Murray F. A large prospective open–label, multi–centre SAMM study, comparing the safety of aceclofenac with diclofenac in patients with rheumatic disease. Eur.J.Rheumatol.Inflam., 2000,17, 1–7.
32. Kokki H., Tuomilehto H., Tuovinen K. Pain management after adenoidectomy with ketoprofen: comparison of rectal and intravenous routes. Br J Anaesth., 2000, 85(6): 836–840.
33. Konstan M.W., Byard PJ., Hoppel C.L., Davis P.B. Effect of high dose ibuprofen in patients with cystic fibrosis // N. Engl. J. Med., 1995, 332: 848-854.
34. La Grenade С., Lee L., Weaver J., et al. Comparison of reporting of Stevens–Johnson Syndrome and toxic epidermal necrosis in association with selective COX–2 inhibitors. Drugs Saf 2005; 28 (10): 917–924.
35. Lai K., Chu K., Hui W. et al. Celecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complications. Am J Med. 2005, 118(11), 1271–1278.
36. Lanas A., Garcia–Rodriguez L., Arroyo M., et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo–oxygenase–2 inhibitors, traditional non–aspirin non–steroidal anti–inflammatory drugs, aspirin and combinations. Gut. 2006, 55(12): 1731–738.
37. Laporte J., Ibanez L., Vidal X., Vendrell L., Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Safety 2004, 27, 411–420.
38. Laurence D.R., Bennett P.N. Clinical Pharmacology. 7th ed. Churcill Livingstone. 1992.
39. Layton D., Hughes K., Harris S., Shakir S. Comparison of the incidence rates of selected ga–strointestinal events reported for patients prescribed celecoxib and meloxicam in general practice in England using prescription–event monitoring (PEM) data. Rheumatology (Oxford). 2003, 42(11): 1332–1341.
40. MacDonald T., Morant S., Goldstein J., et al. Channelling bias and the incidence of gastrointestinal haemorrhage in users of meloxicam, coxibs, and older, non–specific non–steroidal anti–inflammatory drugs. Gut. 2003, 52(9): 1265–1270.
41. Malmstrom K., Fricke J., Kotey P., et al. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single–center, randomized, double–blind, placebo– and active–comparator–controlled, parallel–group, single–dose study using the dental impaction pain model. Clin Ther. 2002, 24(10):1549–1560.
42. Mamdani M., Juuurkin D., Lee D., et al. Cyclo–oxygenase–2 inhibitors versus non–selective non–steroidal anti–inflammatory drugs and congestive heart failure outcomes in eldery patients: a population–based cohort study. Lancet 2004; 363: 1751–1756.
43. McGettigan P., Henry D. Cardiovascular risk and inhibition of cyclooxygenase. JAMA, 2006, 296, 1633–1644.
44. Mellemkjaer L., Blot W., Sorensen H., et al. Upper gastrointestinal bleeding among users of NSAIDs: a population–based cohort study in Denmark. Br J Clin Pharmacol., 2002, 53, 173–181.
45. Moore N. Diclofenac potassium 12,5mg tablets for mild to moderate pain and fever: a re–view of its pharmacology, clinical efficacy and safety. Clin Drug Investig. 2007;27(3):163–195.
46. Moore R., Derry S., Makinson G., McQuay H. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systemic review and meta–analysis on information from company clinical reports. Arthritis Res Ther 2005; 7: 644–665.
47. Neighbor M., Puntillo K. Intramuscular ketorolac vs oral ibuprofen in emergency department patients with acute pain. Acad Emerg Med. 1998,5(2): 92–93.
48. Noble S, Balfour J. Meloxicam // Drugs, 1996, 51: 424-430.
49. O’Donnell J., Ekman E., Spalding W., McCabe D. Analgesic effectiveness, tolerability, and safety of celecoxib versus tramadol in patients with chronic low back pain. ACR, 2007.
50. Perneger T.V., Whelton P.К., Klag MJ. Risk of kidney failure associated with the use of acetaminophen, aspirin, and no steroidal anti-inflammatory drugs // N. Engl. J. Med, 1994, 331: 1675-1712.
51. Pincus T., Koch G., Lei H., et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann. Rheum. Dis., 2004, 63, 931–939.
52. Pinto Pereira L., Chen D., Clement Y., Simeon D. Analgesic effects of diclofenac suppository and injection after preoperative administration. Int J Clin Pharmacol Res. 1999;19(2):47–51.
53. Pochobrasky M., Mele G. et ak. Post-marketing Survey of nimesulide in the Shontterm treatment of osteoarthritis. Drug Exp. Clin. Res., 1991; 17: 197-204/
54. Prado J., Daza R., Chumbes O., et al. Antipyretic efficacy and tolerability of oral ibuprofen, oral dipyrone and intramuscular dipyrone in children: a randomized controlled trial. Sao Paulo Med J. 2006, 124(3): 135–140.
55. Rabasseda X. Safety profile of nimesulide: ten years of clinical experience. Drug of Today, 1997; 33:1-11.
56. Radhofer–Welte S., Dittrich P., Simin M., Branebjerg P. Comparative bioavailability of lornoxicam as single doses of quick–release tablet, standard tablet and intramuscular injection: a randomized, open–label, crossover phase I study in healthy volunteers. Clin Drug Investig. 2008;28(6):345–351.
57. Rahme E., Bardou M., Dasgupta K., et al. Hospitalization for gastrointestinal bleeding associated with non–steroidal anti–inflammatory drugs among elderly patients using low–dose aspirin: a retrospective cohort study. Rheumatology (Oxford). 2007, 46(2): 265–272.
58. Scheiman J., Yeomans N., Talley N., et al. Prevention of ulcer by esomeprazole in at–risk patients using non–selective NSAIDs or COX–2 inhibitor. Am. J. Gastroenterol., 2006, 101, 701–710.
59. Schnitzer T., Weaver A., Polis A. Efficacy of rofecoxib, celecoxib, and acetaminophen in patients with osteoarthritis of the knee. A combined analysis of the VACT studies. J. Rheumatol., 2005, 32, 1093–1105.
60. Senna G., Passalacqua G., Dama A., et al. Nimesulide and meloxicam are a safe alternative drugs for patients intolerant to nonsteroidal anti–inflammatory drugs. Eur Ann Allergy Clin Immunol. 2003, 35(10), 393–396.
61. Silverstein F., Faich G., Goldstein J., et al. Gastrointestinal toxicity with celecoxib versus nonsteroidal anti–inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxid long–term arthritis safety study. JAMA 2000; 84: 1247–1255.
62. Simon L., Weaver A., Graham D. Anti–inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized control trial. JAMA 1999; 282;1921–1928.
63. Singh G, Fort J, Goldstein J. et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS–1 study. Am J Med 2006; 119: 255–266.
64. Singh G, Lanes S, Triadafilopoulos G. Risk of serious upper gastrointestinal and cardiovascular thromboembolic cpmplications with meloxicam. Am J Med 2004; 117: 100–106.
65. Singh G., Graham D., Wang H., et al. Concominant aspirin use reduces the risk of acute myocardial infarction in users of cyclooxygenase–2 selective and some non–selective non–steroidal anti–inflammatory drugs. Ann Rheum Dis 2006; 65 (Suppl. II): 61 (OP0024 abst).
66. Singh G., Mithal A., Triadafilopoulos G. Both selective COX–2 inhibitors and non–selective NSAIDs increase the risk of acute myocardial infarction in patients with arthritis; selectivity is with patients, not the drug. Ann Rheum Dis 2005, 64 (suppl 3), 85.
67. Song I., Poddubnyy D., Rudwaleit M., Sieper J. Benefits and Risks of Ankylosing Spondylitis Treatment With Nonsteroidal Antiinflammatory Drugs. Arthritis & Rheum, 2008, 58 (4), 929–938.
68. Sowers J., White W., Pitt B., et al. The Effects of cyclooxygenase–2 inhibitors and nonsteroidal anti–inflammatory therapy on 24–hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med. 2005 Jan 24;165(2):161–168.
69. Strand V. Are COX–2 inhibitors preferable to non–selective non–steroidal anti–inflammatory drugs in patients with risk of cardiovascular events taking low–dose aspirin? Lancet. 2007, 22; 370(9605), 2138–2151.
70. Van Nieuwenhove K., Van Herreweghe I., Haentjens M., et al. Ulcers of the rectal mucosa caused by suppositories containing acetylsalicylic acid. J Pediatr Gastroenterol Nutr. 1997, 25(4): 430–431.
71. Wanders A., Heijde D., Landewe R., et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum., 2005, 52, 1756–1765.
72. Warner T. D. Introduction to «Nimesulid: beyond COX-2». Clin Exp. Rheumatol, 2001; 19 (Suppl. 22): S1-S2.
73. White W., Kent J., Taylor A., et al. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension. 2002, 39(4): 929–934.
74. White W., West C., Borer J., et al. Risk of cardiovascular events in patients receiving celecoxib: a meta–analysis of randomized clinical trials. Am J Cardiol. 2007, 99(1): 91–98.
75. Wright J., Price S., Watson W. NSAID use and efficacy in the emergency department: single doses of oral ibuprofen versus intramuscular ketorolac. Ann Pharmacother. 1994, 28(3): 309–312.
Date: 2015-07-01; view: 520; Нарушение авторских прав