
Полезное:
Как сделать разговор полезным и приятным
Как сделать объемную звезду своими руками
Как сделать то, что делать не хочется?
Как сделать погремушку
Как сделать так чтобы женщины сами знакомились с вами
Как сделать идею коммерческой
Как сделать хорошую растяжку ног?
Как сделать наш разум здоровым?
Как сделать, чтобы люди обманывали меньше
Вопрос 4. Как сделать так, чтобы вас уважали и ценили?
Как сделать лучше себе и другим людям
Как сделать свидание интересным?

Категории:
АрхитектураАстрономияБиологияГеографияГеологияИнформатикаИскусствоИсторияКулинарияКультураМаркетингМатематикаМедицинаМенеджментОхрана трудаПравоПроизводствоПсихологияРелигияСоциологияСпортТехникаФизикаФилософияХимияЭкологияЭкономикаЭлектроника

Batteries
|
|
Batteries as sources of electrical energy are the result of a long series of experiments which started with the discoveries of Alessandro Volta, an Italian scientist, more than one hundred years ago. Today battery cells are manufactured in two common forms: (I) dry cells, used in flashlights, portable radios, etc., and (2) wet cells, used in automobiles, airplanes, boats, etc. The voltaic cell, Fig. 9. The diagram of voltaic cell as shown in Fig. 9, is composed of three parts, a pair of dissimilar metal plates called electrodes, a dilute acid solution called the electrolyte, and a nonconducting container called the cell.
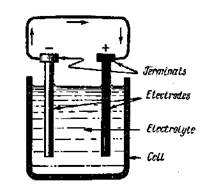
In a glass container filled with sulphuric acid there are two plates: one copper and the other zinc. If the two plates are connected by a copper wire, electricity will flow through
it from the copper plate to the zinc plate. This may be shown by the wire becoming hot. If an ammeter is connected between the plates or electrodes, as they are now called, it will indicate that an electric current is flowing.
The electrode from which electricity flows is termed the positive electrode and the receiving electrode is termed the negative electrode. Thus for the voltaic cell the copper plate is the positive electrode and the zinc plate the negative electrode. A copper wire will convey electricity and is called an electrical conductor. Copper, aluminium and silver are outstandingly good conductors. Conductors must be surrounded by protective material which does not conduct electricity and prevent it to leak away. Materials which do not conduct electricity are called electrical insulators; there are many common examples — glass, wood, rubber, some plastics, "insulation" tape.
Remember that faulty insulation is dangerous and leads to unwanted electrical flow and probably to local overheating.
One common form of cell is shown in Fig. 9. If two or more cells are connected together, they form what is called a battery (Fig. 10). Fig. 10. The diagram of a battery
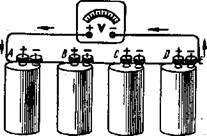
In this diagram the battery is composed of four dry cells connected in series. By series connection it is meant that the (+) terminal of one cell is connected to the (-) terminal of the next. The purpose in connecting two or more cells in series is to obtain a higher emf than that available with one cell alone.
Each cell produces an emf of 1.5 volts, so that if the voltmeter is connected to the points, it will indicate 1.5 volts between A and B, 30 volts between A and C, 4.5 volts between A and D and 6 volts between A and E.
The common flashlight contains several dry cells connected in series.
Date: 2015-10-21; view: 1416; Нарушение авторских прав