
Полезное:
Как сделать разговор полезным и приятным
Как сделать объемную звезду своими руками
Как сделать то, что делать не хочется?
Как сделать погремушку
Как сделать так чтобы женщины сами знакомились с вами
Как сделать идею коммерческой
Как сделать хорошую растяжку ног?
Как сделать наш разум здоровым?
Как сделать, чтобы люди обманывали меньше
Вопрос 4. Как сделать так, чтобы вас уважали и ценили?
Как сделать лучше себе и другим людям
Как сделать свидание интересным?

Категории:
АрхитектураАстрономияБиологияГеографияГеологияИнформатикаИскусствоИсторияКулинарияКультураМаркетингМатематикаМедицинаМенеджментОхрана трудаПравоПроизводствоПсихологияРелигияСоциологияСпортТехникаФизикаФилософияХимияЭкологияЭкономикаЭлектроника

Tyler N. Graf f, and
8. Nicholas H. Oberliesf
+ Author Affiliations
1. Departments of aLaboratory Medicine, 2. bMicrobiology and 3. cGlobal Health, University of Washington, Seattle, WA 98104; 4. dDepartment of Molecular Virology, University of Heidelberg, 69120 Heidelberg, Germany; 5. eBio-Organic and Natural Products Laboratory, McLean Hospital, Belmont, MA 02478; and 6. fDepartment of Chemistry and Biochemistry, University of North Carolina at Greensboro, Greensboro, NC 274021. Edited* by Harvey J. Alter, The Warren G. Magnuson Clinical Center, Bethesda, MD, and approved February 22, 2010 (received for review December 11, 2009)
Next Section
Abstract
Silymarin, also known as milk thistle extract, inhibits hepatitis C virus (HCV) infection and also displays antioxidant, anti-inflammatory, and immunomodulatory actions that contribute to its hepatoprotective effects. In the current study, we evaluated the hepatoprotective actions of the seven major flavonolignans and one flavonoid that comprise silymarin. Activities tested included inhibition of: HCV cell culture infection, NS5B polymerase activity, TNF-α-induced NF-κB transcription, virus-induced oxidative stress, and T-cell proliferation. All compounds were well tolerated by Huh7 human hepatoma cells up to 80 μM, except for isosilybin B, which was toxic to cells above 10 μM. Select compounds had stronger hepatoprotective functions than silymarin in all assays tested except in T cell proliferation. Pure compounds inhibited JFH-1 NS5B polymerase but only at concentrations above 300 μM. Silymarin suppressed TNF-α activation of NF-κB dependent transcription, which involved partial inhibition of IκB and RelA/p65 serine phosphorylation, and p50 and p65 nuclear translocation, without affecting binding of p50 and p65 to DNA. All compounds blocked JFH-1 virus-induced oxidative stress, including compounds that lacked antiviral activity. The most potent compounds across multiple assays were taxifolin, isosilybin A, silybin A, silybin B, and silibinin, a mixture of silybin A and silybin B. The data suggest that silymarin- and silymarin-derived compounds may influence HCV disease course in some patients. Studies where standardized silymarin is dosed to identify specific clinical endpoints are urgently needed.
· hepatitis C
· liver disease
· milk thistle
· botanical medicine
· hepatoprotection
Chronic hepatitis C virus (HCV) is a major global medical problem. In the United States, millions of people are affected, the number of patients with HCV-induced end-stage liver disease is growing (1), and this condition is already the leading indication for liver transplantation (2). The current standard of care for chronic hepatitis C, pegylated IFN-α and ribavirin, results in sustained elimination of virus in 55% of treated patients (3, 4). However, significant numbers of patients do not clear the virus and are intolerant to, have contraindications to, or opt out of therapy. Furthermore, because emerging specifically targeted antiviral therapy for HCV therapies need to be administered with pegylated IFN plus ribavirin (5), it is likely that many patients will not tolerate this therapy. Thus, there are many patients who have no other Food and Drug Administration-approved options to eliminate HCV and prevent progression of liver disease. As a result, many individuals have opted for complementary and alternative medicine-based approaches, including botanicals, to treat their chronic hepatitis C. Indeed, as many as 13 to 23% of American patients with chronic liver disease use botanical medicines, with silymarin being the most popular (6, 7).
Silymarin, an extract from the seeds of the milk thistle plant, Silybum marianum, has been used for centuries as a hepatoprotectant. Although silymarin has been described to possess antioxidant, immunomodulatory, antiproliferative, antifibrotic, and antiviral activities (8, 9), its mechanisms of action remain to be fully defined. Although its clinical efficacy is currently uncertain (8, 10), interest in this botanical medicine has been piqued by studies showing silymarin blocks HCV cell culture (HCVcc) infection (9). Intravenous administration of silibinin, composed of a 1:1 mixture of silybin A and silybin B, causes dose-dependent reduction of viral load in patients with chronic hepatitis C (11).
We previously showed that silymarin blocked HCVcc infection of human hepatoma cultures, inhibited TNF-α and TCR induced NFκB-dependent transcription, and suppressed TCR-mediated proliferation and inflammatory cytokine production from T cells (9, 12). Thus, silymarin displays antiviral, anti-inflammatory, and immunomodulatory functions in human liver and immune cells. We (13) and others (14) have recently shown that silymarin can also block in vitro HCV NS5B polymerase activity at high concentration. Therefore, in the current study, we screened the seven major flavonolignans and one flavonoid that comprise silymarin for antiviral, antipolymerase, anti-NF-κB, and immunomodulatory actions. In addition, we assessed the antioxidant potential of the pure compounds.
Previous SectionNext Section
Results
Silymarin is a complex of eight major compounds, including seven flavonolignans: silybin A, silybin B, isosilybin A, isosilybin B, silychristin, isosilychristin, silydianin, and one flavonoid, taxifolin (15). Compounds were purified as previously described (16) (Fig. S1). We then tested the effects of silymarin and pure compounds in assays for HCVcc infection, NS5B polymerase activity, HCVcc-induced oxidative stress, TNF-α-induced NF-κB transcription, and TCR-mediated induction of T-cell proliferation.
We first performed cytotoxicity dose-response experiments by measuring ATP levels, a sensitive marker of cell viability. All compounds were well tolerated by Huh7.5.1 human hepatoma cells up to 80 μM, except for isosilybin B, which was toxic to cells above 10 μM (Fig. S2). Silydianin, silychristin, isosilychristin, and taxifolin were not toxic at 100 μM.
Fig. 1 A depicts the antiviral effects of the pure compounds against in vitro HCV infection, based on HCV protein expression. The concentrations that inhibited HCV protein by 50% (IC50) are shown in Table 1. Taxifolin, isosilybin A, and silybin A were the most effective in inhibiting JFH-1 infection, and these compounds were more potent than silymarin extract. Silybin B and silibinin, a 1:1 mixture of silybin A and silybin B, also inhibited HCV infection with potencies similar to silymarin. Isosilychristin, silydianin, and isosilybin B did not inhibit HCV protein expression.
View this table:
· In this window
· In a new window
Table 1.
Hepatoprotective effects of silymarin-derived flavonolignans

View larger version:
· In this page
· In a new window
· Download as PowerPoint Slide
Fig. 1.
Antiviral effects of silymarin-derived flavonolignans. (A) Effects on HCV protein expression. JFH-1 virus at a multiplicity of infection of 0.05 was adsorbed to Huh7.5.1 cells for 5 h. After the inoculum was removed, fresh medium was added containing DMSO or 20.7, 41.4, 82.8, or 124.2 μM of pure flavonolignan (mw 482), or 32.9, 65.8, 131.6, or 197.4 μM of taxifolin (mw 304). Protein lysates were harvested 72 h later and HCV protein expression detected by Western blot analysis. Actin served as a loading control. (B) Effects on HCV RNA. JFH-1 virus at a multiplicity of infection of 0.05 was adsorbed to Huh7.5.1 cells for 5 h. After the inoculum was removed, fresh medium was added containing DMSO or pure flavonolignan or taxifolin at the concentrations listed above. Total RNA was harvested 72 h later and HCV RNA expression quantitified by real-time RT-PCR. The y axis presents the number of copies of HCV RNA per 10 ng of total RNA. Silydianin (SD), silychristin (SC), isosilychristin (ISC), silybin A (SA), and silybin B (SB), taxifolin (TAX), isosilybin A (ISA), isosilybin B (ISB), silibinin (SbN), and silymarin (SM).
Fig. 1 B and Table 1 summarize inhibition of HCV RNA replication among the different compounds. Taxifolin was the most potent at blocking HCV RNA replication, followed by isosilybin A, silybin B, silybin A, silibinin, and silychristin, which were all more potent than silymarin. Silydianin and isosilychristin were inactive.
Silymarin inhibits HCV NS5B polymerase activity, albeit at concentrations 5- to 10-fold higher than required for HCVcc inhibition (13). We therefore tested the pure compounds for their ability to inhibit JFH-1 NS5B polymerase activity. NS5B polymerase inhibition was only observed at very high concentrations, with silymarin and silybin B displaying the most potent activities, with IC50 values of 300 to 600 μM (Table 1).
In summary, the data indicate that certain silymarin-derived pure flavonolignans can inhibit HCV infection as measured by blockade of HCV protein and RNA expression, but that inhibition of NS5B RdRp activity can only partly explain the antiviral activity. Indeed, we have shown that silymarin blocks virus entry and fusion, and virus production (13).
To determine if silymarin contains antioxidant functions in the context of HCV infection, we treated infected cells with silymarin, then labeled cells with the oxidant reactive dye, H2DCFDA, and measured fluorescence by microscopy. JFH-1 infection of Huh7.5.1 cells caused large increases in fluorescently labeled cells compared with mock-infected cells, indicative of oxidative stress (Fig. 2 A). Treatment of cells with three different preparations of silymarin (MK-001, USP, and Legalon) caused significant reductions of cellular fluorescence (Fig. S3). Treatment with all eight pure compounds inhibited JFH-1-induced oxidative stress (Fig. 2 B). IFN, a known antiviral for HCV, also inhibited JFH-1-induced oxidative stress. Silibinin (a 1:1 mixture of silybin A and silybin B), taxifolin, and isosilybin A had antioxidant activity equal to or greater than silymarin, causing greater than 90% reduction in JFH-1-induced oxidative stress. Compounds that lacked antiviral action, such as silydianin and isosilychristin, still possessed antioxidant function.
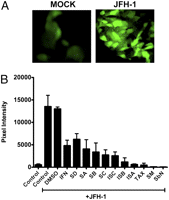
View larger version:
· In this page
· In a new window
· Download as PowerPoint Slide
Fig. 2.
Silymarin and silymarin-derived flavonolignans block HCV induced oxidative stress. (A) Huh7.5.1 cells were infected or mock-infected with JFH-1 at a multiplicity of infection of 0.01 and 72 h later, cells were labeled with the dye H2DCFDA for 30 min using the Image-IT Live Green Reactive Oxygen Species kit (Molecular Probes/Invitrogen). On oxidation, H2DCFDA becomes highly fluorescent. Images were captured on a Nikon Microscope using MetaMorph software. (B) Huh7.5.1 cells were treated as described above except in addition to silymarin (SM), cells were also treated with 41.4 μM of each flavonolignan, 65.8 μM of taxifolin, or 100 U/mL of IFN-α. ROS were quantitated by pixel intensity. Abbreviations of compounds are listed in the legend to Fig. 1.
NF-κB is the key transcription factor that is central to the induction of an inflammatory response (17). NF-κB transcription factors are homo- and heterodimeric complexes of proteins with molecular weights of 50 and 65 kDa (18). Inactive NF-κB is found in the cytoplasm via its association with inhibitory proteins of the IκB family. Stimulation of NF-κB activity involves phosphorylation of IκB-α on serine amino acid 32, phosphorylation of NF-κB on serine 536, followed by degradation of IκB-α, permitting NF-κB translocation to the nucleus and expression of inflammatory response genes (19, 20).
Silymarin blocks NF-κB-dependent transcription from a canonical NF-κB promoter (9). Silymarin also blocked TNF-α activation of NF-κB dependent transcription from the positive regulatory domain (pRDII) domain of the IFN-B promoter, a key gene in antiviral defense (21) (Fig. S4 A). Silymarin reduced both p65 and IκB-α serine phosphorylation by about 20% and caused an approximate 20% decrease in TNF-α induced p50 and p65 nuclear translocation (Fig. S4 C and D). However, silymarin did not affect the ability of p50 or p65 to bind to the NF-κB promoter (Fig. S4 E). The data suggest that silymarin partially inhibits phosphorylation of IκB-α and p65/RelA and nuclear translocation of p50 and p65 subunits of NF-κB, but does not impair the ability of NF-κB to bind to DNA.
Fig. 3 depicts examples of the effects on the pure compounds’ abilities to block TNF-α induced NF-κB-dependent transcription. In this assay, we were able to test higher concentrations of silymarin because the flavonolignans were only incubated with cells for 4 h. Silybin A and silybin B caused dose-dependent blockade of TNF-α induced NF-κB dependent transcription, with IC50s of 40 μM (Fig. 3 A and B and Table 1). Compared with silybin A and B, taxifolin induced the most-pronounced inhibition of NF-κB transcription at the lowest doses, but its effect appeared to plateau (Fig. 3 C).

View larger version:
· In this page
· In a new window
· Download as PowerPoint Slide
Fig. 3.
Silymarin flavonolignans inhibit NF-κB transcription. (A and B) Silybin A and silybin B inhibit NF-κB transcription. Huh7 cells were transfected with a luciferase reporter gene under control of the pRDII domain from the IFN-B promoter and 24 h later, cells were treated with the indicated micromolar doses of each flavonolignan or DMSO control for 30 min before addition of 10 ng/mL TNF-α. Luciferase activity was measured 3.5 h later. Error bars represent SDs. (C) Comparison of NF-κB inhibitory profiles of silybin A, silybin B, taxifolin, and silychristin.
Table 1 summarizes the effects of silymarin at 40 μM on T-cell proliferation induced by CD3 ligation. Silymarin, silibinin, silybin A, and isosilybin A displayed the most potent suppression of T-cell proliferation. Isosilychristin, silydianin, and taxifolin were essentially inactive. Isosilybin B also blocked T-cell proliferation, but this was because of toxicity. In contrast to the other measures of hepatoprotection, where some pure compounds were more active than silymarin, silymarin was the most potent in blocking T-cell proliferation. Silymarin and pure compounds also inhibited inflammatory cytokine expression from T cells (Fig. S5).
Previous SectionNext Section
Discussion
To our knowledge, this report is unique in characterizing silymarin and silymarin-derived pure compounds in four different measures of hepatoprotection in human cells and in the context of hepatitis C virus infection. The natural compounds inhibit virus infection, virus-induced oxidative stress, NF-κB-dependent transcription, and TCR mediated proliferation. Table S1 summarizes the data of compound activity in the five hepatoprotective assays. Isosilybin A, taxifolin, and silibinin were the most effective hepatoprotectors because they displayed potent activity in four of five assays, followed by silybin A and silybin B, which were active in three of five assays. Taxifolin was particularly noteworthy because hepatoprotection was observable at lower doses than the other compounds, and tended to plateau after reaching a nadir at low doses. In contrast, the other compounds showed more linear dose-response relationships. Intriguingly, all compounds inhibited virus-induced oxidative stress regardless of whether they had antiviral activity.
With the exception of T cell proliferation, several purified compounds were more potent than the parent silymarin extract. Although some complementary and alternative medicine practioners may emphasize that it is the combination of bioactive molecules in a botanical extract that defines its unique biological properties, our data suggest that combinations of flavonolignans may provide optimal hepatoprotection. Because the structures of the flavonolignan stereoisomers are similar, there may be competitive interactions between flavonolignans for cellular targets. This possibility implies that the hepatoprotective potential of select mixtures of pure compounds could in theory be greater than the silymarin extract itself. Moreover, different combinations of flavonolignans might be selected, depending on the hepatoprotective functions one is targeting. Given that isosilybin B is very toxic in cell culture, as shown here, select combinations may be especially relevant if one is trying to minimize cytotoxicity for treatment of liver diseases of both viral and nonviral origin. Further studies that examine combinations and define the cellular targets of the pure compounds are needed to resolve these issues.
In the current study, although silymarin and silybin B appeared to be the most potent polymerase inhibitors, the IC50 concentrations were much higher than the concentrations that caused cytotoxicity. Moreover, silymarin displays weak polymerase inhibition against four clinical isolates of genotype 1b HCV RdRps, and silymarin does not inhibit HCV replication in noninfectious replicon cell lines (13). Therefore, we propose that polymerase inhibition by the natural compounds, although demonstrable in vitro, may not contribute to anti-HCVcc action in vitro, and plays a limited role in antiviral effects in patients with chronic hepatitis C when silymarin is taken orally. However, the i.v. formulation of silibinin that displays antiviral effects, Legalon-SIL, is actually the dihydrogen disuccinate disodium versions of silybin A and silybin B. This chemical modification makes silibinin water soluble, so this formulation may have a different antiviral profile and mechanism of action than the natural compounds.
NF-κB serine phosphorylation is required for maximal transcriptional activation by NF-κB (22). We showed that silymarin partially inhibits NF-κB-dependent transcription via partial blockade of phosphorylation of Ser536 on the p65 subunit of NF-κB and serine32 on IκB-α. Silymarin also partially inhibited nuclear translocation of the p50 and p65 NF-κB subunits. These results are in agreement with previous studies in prostate cancer cells, where silibinin was shown to block p50/p65 nuclear translocation and IKK-α activity (23).
HCV infection induces oxidative stress and inflammation (24–27). Unchecked oxidative stress can modify proteins and lipids, damage DNA, alter mitochondrial membrane potential, and be a predisposing event in the progression of liver disease. In the present study, we demonstrated that JFH-1 infection caused large increases in oxidative stress. These effects were abrogated by silymarin and pure compound treatment. We suggest that silymarin inhibits HCV-induced oxidative stress by at least two mechanisms. First, silymarin's demonstrated antiviral actions could have reduced HCV replication and the ensuing oxidative stress. Second, flavonolignan components of silymarin may have direct antioxidant functions without antiviral functions. Evidence in support of both modes of action was provided in the present study. These data extend silymarin's previously documented antioxidant functions (28) to the context of HCV infection. Because silymarin inhibits oxidative stress independently of suppression of virus, it is possible that the hepatoprotective actions of the botanical may be extended to liver diseases of nonviral origin.
Although silibinin, which is a mixture of silybin A and silybin B, has been largely touted to contain many of the hepatoprotective functions (28, 29), we are unique in showing that there are other flavonolignans in silymarin that are equal to, if not more potent, than silibinin. The best examples are isosilybin A and taxifolin, which have antiviral activities that were superior to silibinin. Yet in T cell proliferation silibinin, but not taxifolin, displayed significant activity. These data underscore the importance of careful evaluation of all flavonolignans and that the activity of each flavonolignan may vary depending on the biological assay.
Our data suggest that silymarin and silymarin-derived compounds could influence HCV disease course in some persons. Although standardized silymarin has an exceptional safety record (8), the effects observed in vitro occur at relatively high concentrations, so additional studies are necessary to assess if adverse effects occur in humans at these levels. Therefore, we caution against the self-administration of large amounts of these botanicals, particularly those purchased as dietary supplements, because there is inconsistency in the standardization of these products, the entire contents may not be known, they may contain contaminants, and misuse of herbals can cause hepatotoxicity (30). On the other hand, the activities observed in the present study using highly pure natural compounds suggests great promise for the treatment of hepatitis C, for which previously unexplored therapeutics are urgently needed.
Silymarin is an interesting botanical extract that has a multitude of biological activities. The four activities we measured: antiviral, antioxidant, anti-inflammatory, and immunomodulatory, are all likely to be directly related to its well-described hepatoprotective actions (31), but seemingly much harder to demonstrate in humans (32). Although the latter may be primarily because of the metabolism and bioavailability of silymarin and a dearth of properly designed clinical studies, it is clear that further studies are necessary to define the molecular mechanisms by which silymarin exerts such pleiotropic effects.
Previous SectionNext Section
Methods
Subjects.
Subjects with and without chronic HCV infection were recruited at Harborview Medical Center after written informed consent was obtained through a University of Washington Institutional Review Board–approved protocol.
PBMC Isolation and Proliferation Assay.
Peripheral blood mononuclear cells (PBMCs) were isolated within 24 h of venipuncture and immediately assayed. PBMC were stimulated for 24 h at 37 °C 5% CO2 using plate-bound anti-CD3 (UCHT1, 10 μg/mL, BD Biosciences) in RPMI medium 1640 supplemented with 10% human serum (Gemini Bio-Products). Cellular proliferation detected by 3H-thymidine incorporation into replicating DNA was measured by adding 1 μCi to each replicate well of 105 PBMC for an additional 24 h before quantitative analysis using a Topcount Liquid Scintillation Counter (Perkin-Elmer). All experiments used four replicates per condition. Data were obtained as mean cpm incorporated per condition tested. Percent inhibition was calculated using the following formula: 100 × (vehicle-treated mean cpm − compound-treated mean cpm)/vehicle-treated mean cpm.
Cells, Virus, and Plasmids.
Huh7.5.1 cells were grown in Huh7 medium as described (9). JFH-1 viral stock preparation, cell infection, and titration was performed as described (33–35). For antiviral assays, cells were in infected at a multiplicity of infection of 0.05 and protein and RNA harvested 72 h postinfection. To measure NF-κB–dependent transcription, we used a luciferase reporter gene under control of the positive regulatory domain (pRDII), which contains the NF-κB site from the IFN-B promoter.
Silymarin Preparations, and Pure Compounds.
Three preparations of silymarin were used in the current study. The first was MK-001, prepared as described (9, 36). The second preparation was commercially prepared (Legalon; Madaus). The third preparation was purchased from US Pharmacopeia. Silymarin was solubilized in DMSO at 50 mg/mL for Huh7 cell studies; methanol was the solvent for PBMC studies. Pure flavonolignans were solubilized at 50 mg/mL in DMSO for all studies except PBMC studies, where methanol was used as the solvent.
Silymarin treatment of Huh7.5.1 cultures consisted of adding silymarin immediately after removing the viral inoculum. For PBMC studies, silymarin or pure compounds were added to cells at the same time that they were exposed to plate-bound anti-CD3 (T-cell receptor antibody).
Cytotoxicity Determinations.
The toxicity of silymarin extracts and pure flavonolignans on Huh7.5.1 cells was determined by measuring ATP levels using the ATPlite system (Perkin-Elmer), as described (9).
Reporter Gene Assays.
Reporter gene assays were performed as described (37). Endotoxin free plasmid DNA was purified (Endofree kit, Qiagen), and was introduced into cells with Lipofectamine 2000 according to manufacturer's recommendations (Invitrogen). Next, 100 ng of the pRDII-luciferase gene was transfected into cells in quadruplicate. Eighteen hours later, cells were preincubated with silymarin or pure compounds for 30 min before rhTNF-α (15 ng/mL; Sigma Aldrich) was added. Four hours later, luciferase activity was measured on cell lysates using the Britelite Assay System (Perkin-Elmer).
Western Blot Analysis.
NS5A and core proteins in JFH-1 infected cells were detected with serum from patients infected with HCV genotype 2a. The HCV core protein was detected with a monoclonal antibody (Affinity BioReagents). GAPDH was detected with polyclonal antiserum (Santa Cruz Biotechnology). NF-κB antibodies consisted of a monoclonal antibody to RelA/p65 Serine 536, IκBα Serine 32 (Cell Signaling), and antibodies to p50 and p65 subunits (Upstate/Millipore). For Western hybridizations and washings, both the standard seal a meal bag approach and the Snap-ID system (Millipore) were used.
Oxidative Stress Measurements.
Huh7.5.1 cells were incubated with 20 μg/mL of silymarin or pure flavonlignans immediately after virus adsorption. Seventy-two hours later, cells were labeled with the dye 2',7'-dichlorodihydrofluorecein diacetate (H2DCFDA) for 30 min using the Image-IT Live Green Reactive Oxygen Species kit (Molecular Probes/Invitrogen). H2DCFDA is a cell-permeable indicator for reactive oxygen species that is nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. On oxidation, H2DCFDA becomes highly fluorescent Pharmacopeia. Images were captured on a Nikon Microscope using MetaMorph software (Molecular Devices) with a 20× objective.
HCV NS5B Polymerase Assays.
NS5BΔC21 C-terminally fused to a hexa-histidine tag was expressed and purified for HCV JFH1 and for the genotype 1b isolates as described (38). JFH-1 RNA dependent RNA polymerase (RdRp) assays contained buffer, 5 μCi of [α- 32P]GTP, 50 μM GTP, 1 mM each ATP, CTP, UTP, 2 μg polyC (GE Healthcare), 1 μg purified polymerase, and given amounts of purified compounds in DMSO in a total volume of 25 μL. All reaction components except nucleotides and template were preincubated for 15 min at room temperature; the reaction was started by adding the nucleotide mixture and polyC and was incubated for 1.5 h at room temperature. Reaction products were precipitated, passed through microfilters (GE Healthcare), washed five times with 1% trichloroacetic acid and 0.1% tetra-sodium pyrophosphate, and air-dried. After addition of 6 mL of Ultima Gold (Perkin-Elmer), samples were subjected to liquid scintillation counting. All measurements were performed in triplicate and the IC50 values were calculated with GraphPad Prism.
Previous SectionNext Section
Acknowledgments
We thank Jessica Wagoner and Minjun Chung for technical assistance. S.J.P. is partially supported by National Institutes of Health Grant AT002895 from the National Center for Complementary and Alternative Medicine. V.L. is supported by the Deutsche Forschungsgemeinschaft, LO1556/1-1.
Previous SectionNext Section
Footnotes
· 1To whom correspondence should be addressed. E-mail: [email protected].
· Author contributions: S.J.P., C.M., and V.L. designed research; S.J.P., C.M., V.L., S.P., D.Y.W.L., Y.L., T.N.G., and N.H.O. performed research; V.L., T.N.G., and N.H.O. contributed new reagents/analytic tools; S.J.P., C.M., V.L., S.P., D.Y.W.L., Y.L., T.N.G., and N.H.O. analyzed data; and S.J.P., C.M., V.L., S.P., D.Y.L., and N.H.O. wrote the paper.
· The authors declare no conflict of interest.
· ↵*This Direct Submission article had a prearranged editor.
· This article contains supporting information online at www.pnas.org/cgi/content/full/0914009107/DCSupplemental.
Previous Section
References
1. ↵
1. Davis GL,
2. Albright JE,
3. Cook SF,
4. Rosenberg DM
(2003) Projecting future complications of chronic hepatitis C in the United States. Liver Transpl 9: 331 – 338.
CrossRefMedlineWeb of Science
2. ↵
1. Alter HJ
(2005) HCV natural history: the retrospective and prospective in perspective. J Hepatol 43: 550 – 552.
CrossRefMedlineWeb of Science
3. ↵
1. Manns MP,
2. et al.
(2001) Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358: 958 – 965.
CrossRefMedlineWeb of Science
4. ↵
1. Fried MW,
2. et al.
(2002) Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347: 975 – 982.
CrossRefMedlineWeb of Science
5. ↵
1. Nelson DR
(2009) Hepatitis C drug development at a crossroads. Hepatology 50: 997 – 999.
CrossRefMedlineWeb of Science
6. ↵
1. Strader DB,
2. et al.
(2002) Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol 97: 2391 – 2397.
CrossRefMedlineWeb of Science
7. ↵
1. Seeff LB,
2. et al.,
3. HALT-C Trial Group
(2008) Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology 47: 605 – 612.
CrossRefMedlineWeb of Science
8. ↵
1. Saller R,
2. Meier R,
3. Brignoli R
(2001) The use of silymarin in the treatment of liver diseases. Drugs 61: 2035 – 2063.
CrossRefMedlineWeb of Science
9. ↵
1. Polyak SJ,
2. et al.
(2007) Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology 132: 1925 – 1936.
CrossRefMedlineWeb of Science
10. ↵
1. Jacobs BP,
2. Dennehy C,
3. Ramirez G,
4. Sapp J,
5. Lawrence VA
(2002) Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med 113: 506 – 515.
CrossRefMedlineWeb of Science
11. ↵
1. Ferenci P,
2. et al.
(2008) Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology 135: 1561 – 1567.
CrossRefMedlineWeb of Science
12. ↵
1. Morishima C,
2. et al.
(2010) Silymarin inhibits in vitro T cell proliferation and cytokine production in hepatitis C virus infection. Gastroenterology 138: 671 – 681.
CrossRefMedlineWeb of Science
13. ↵
1. Wagoner J,
2. et al.
(2010) Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology, 10.1002/hep.23587.
14. ↵
1. Ahmed-Belkacem A,
2. et al.
(2009) Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology, 138:1112-1122.
15. ↵
1. Kroll DJ,
2. Shaw HS,
3. Oberlies NH
(2007) Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 6: 110 – 119.
Abstract/FREE Full Text
16. ↵
1. Graf TN,
2. Wani MC,
3. Agarwal R,
4. Kroll DJ,
5. Oberlies NH
(2007) Gram-scale purification of flavonolignan diastereoisomers from Silybum marianum (milk thistle) extract in support of preclinical in vivo studies for prostate cancer chemoprevention. Planta Med 73: 1495 – 1501.
CrossRefMedlineWeb of Science
17. ↵
1. Karin M
(1998) The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am 4 (Suppl 1): S92 – S99.
MedlineWeb of Science
18. ↵
1. Tato CM,
2. Hunter CA
(2002) Host-pathogen interactions: subversion and utilization of the NF-kappa B pathway during infection. Infect Immun 70: 3311 – 3317.
FREE Full Text
19. ↵
1. Mukaida N
(2000) Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol 72: 391 – 398.
MedlineWeb of Science
20. ↵
1. Sakurai H,
2. Chiba H,
3. Miyoshi H,
4. Sugita T,
5. Toriumi W
(1999) IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem 274: 30353 – 30356.
Abstract/FREE Full Text
21. ↵
1. Kanto T
(2008) Virus associated innate immunity in liver. Front Biosci 13: 6183 – 6192.
MedlineWeb of Science
22. ↵
1. Chen LF,
2. Greene WC
(2004) Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392 – 401.
CrossRefMedlineWeb of Science
23. ↵
1. Dhanalakshmi S,
2. Singh RP,
3. Agarwal C,
4. Agarwal R
(2002) Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene 21: 1759 – 1767.
CrossRefMedlineWeb of Science
24. ↵
1. Wen F,
2. et al.
(2004) Increased prooxidant production and enhanced susceptibility to glutathione depletion in HepG2 cells co-expressing HCV core protein and CYP2E1. J Med Virol 72: 230 – 240.
CrossRefMedlineWeb of Science
25. ↵
1. Lai MMC
(2002) Hepatitis C virus proteins: direct link to hepatic oxidative stress, steatosis, carcinogenesis and more. Gastroenterology 122: 568 – 571.
CrossRefMedline
26. ↵
1. Okuda M,
2. et al.
(2002) Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 122: 366 – 375.
CrossRefMedlineWeb of Science
27. ↵
1. Gong G,
2. Waris G,
3. Tanveer R,
4. Siddiqui A
(2001) Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA 98: 9599 – 9604.
Abstract/FREE Full Text
28. ↵
1. Comelli MC,
2. Mengs U,
3. Schneider C,
4. Prosdocimi M
(2007) Toward the definition of the mechanism of action of silymarin: activities related to cellular protection from toxic damage induced by chemotherapy. Integr Cancer Ther 6: 120 – 129.
Abstract/FREE Full Text
29. ↵
1. Gazák R,
2. Walterová D,
3. Kren V
(2007) Silybin and silymarin—new and emerging applications in medicine. Curr Med Chem 14: 315 – 338.
CrossRefMedlineWeb of Science
30. ↵
1. Seeff LB
(2009) Are herbals as safe as their advocates believe? J Hepatol 50: 13 – 16.
CrossRefMedlineWeb of Science
31. ↵
1. Rainone F
(2005) Milk thistle. Am Fam Physician 72: 1285 – 1288.
MedlineWeb of Science
32. ↵
1. Saller R,
2. Brignoli R,
3. Melzer J,
4. Meier R
(2008) An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed 15: 9 – 20.
CrossRefMedline
33. ↵
1. Wakita T,
2. et al.
(2005) Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11: 791 – 796.
CrossRefMedlineWeb of Science
34. ↵
1. Zhong J,
2. et al.
(2005) Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA 102: 9294 – 9299.
Abstract/FREE Full Text
35. ↵
1. Koo BC,
2. et al.
(2006) Relationships between hepatitis C virus replication and CXCL-8 production in vitro. J Virol 80: 7885 – 7893.
Abstract/FREE Full Text
36. ↵
1. Lee DY,
2. Liu Y
(2003) Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, Isolated from Silybum marianum (milk thistle) J Nat Prod 66: 1171 – 1174.
Medline
37. ↵
1. Wagoner J,
2. et al.
(2007) Regulation of CXCL-8 (interleukin-8) induction by double-stranded RNA signaling pathways during hepatitis C virus infection. J Virol 81: 309 – 318.
Abstract/FREE Full Text
38. ↵
1. Binder M,
2. et al.
(2007) Identification of determinants involved in initiation of hepatitis C virus RNA synthesis by using intergenotypic replicase chimeras. J Virol 81: 5270 – 5283.
Abstract/FREE Full Text
·  CiteULike
CiteULike
·  Complore
Complore
·  Connotea
Connotea
·  Delicious
Delicious
·  Digg
Digg
·  Facebook
Facebook
·  Twitter
Twitter
·  Mendeley
Mendeley
What's this?
HighWire Press-hosted articles citing this article
· Investigating the Potential for Toxicity from Long-Term Use of the Herbal Products, Goldenseal and Milk Thistle Toxicol Pathol 2011 39 (2) 398-409
o Abstract
o Full Text
o Full Text (PDF)
Advertisement
· J Lipid Res. 2000 Dec;41(12):1969-79 Expand+
Modulation of hepatic lipoprotein synthesis and secretion by taxifolin, a plant flavonoid1
1. Andre Theriault2, *,
2. Qi Wang*,
3. Stephen C. Van Iderstine†,
4. Biao Chen†,
Date: 2015-09-18; view: 208; Нарушение авторских прав; Помощь в написании работы --> СЮДА... |